Abstract
Background:
Emerging treatments for relapsed/refractory Chronic Lymphocytic Leukemia (CLL) and Mantle Cell Lymphoma (MCL) have improved outcomes and transformed care for most patients with these malignancies. However, the multitude of treatment options and complexities associated with these B-cell malignancies complicate provider-patient communication and limit patient education. To better understand educational gaps and inform the development of practical tools and interventions, this study aimed to identify current challenges faced by Oncologists, Hematologists, and Hemato-Oncologists (ONC/HEM) treating patients with relapsed/refractory CLL and MCL in France, Germany, and the United States (US). Specifically, we focused on challenges encountered in patient-provider communication and shared decision-making for treatment planning and adherence.
Study design and methods:
A quantitative survey was designed and distributed to purposively sampled ONC/HEM from academic (France, Germany, and the US) and community (Germany and US) settings. French specialists were solely from academic settings due to local healthcare structure. The protocol was approved by an independent ethics review board, and participants were compensated for their time. Sampling criteria included ONC/HEM who spent 50%+ time in patient care, had 2+ years post-residency experience, treated ≥5 CLL and ≥2 MCL patients in the last 2 years, and prescribed BTK inhibitors to CLL/MCL patients. Survey items were informed by literature and qualitative interviews with US ONC/HEM (n=6). Confidence questions were asked on 5-point scale, which was recoded as a dichotomic variable for analysis purposes: sub-optimal (if not, slightly, or somewhat confident) or optimal (if confident or very confident). Frequency questions were asked on a 5-point scale (never, rarely, sometimes, often, always). Chi-square tests were calculated to identify differences between the 5 sub-groups, based on country and setting. Results were interpreted by educational experts and clinical faculty specialized in CLL/MCL.
Results:
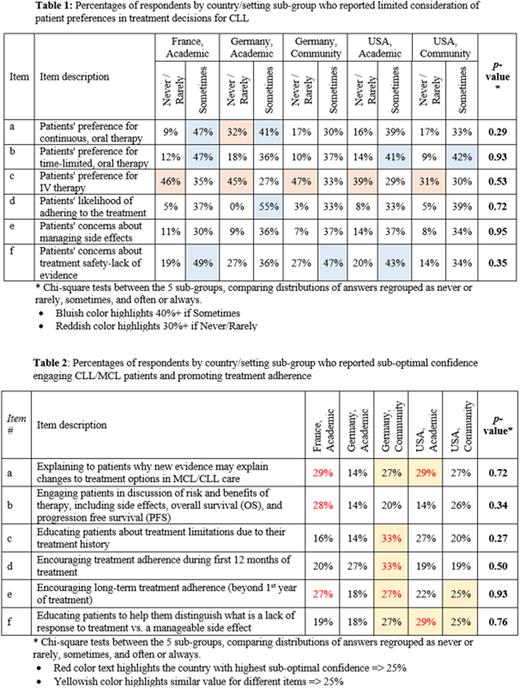
Survey respondents (n=224) were divided between the five subgroups: France academic (n=57), Germany academic (n=22), Germany community (n=30), US academic (n=50), and US community (n=65). Overall, more than a quarter of ONC/HEMs (29%) reported never, rarely, or sometimes considering CLL patients' preferences when recommending a specific therapy. When comparing consideration of patient preferences in selecting a therapy, preferences for IV therapy were least considered, compared to continuous or time-limited oral therapy (Table 1a-c). Half of ONC/HEMs (50%) reported never, rarely, or sometimes considering patients' situations beyond their disease state during treatment decisions; among the 3 items surveyed (Table 1d-f), the least consideration was given to patients' concerns about treatment safety and/or lack of evidence, compared to likelihood of adherence or concerns about managing side effects.
About a quarter of ONC/HEMs (23%) reported sub-optimal confidence educating patients or promoting treatment adherence. One-third of ONC/HEMs (33%) in Germany's community (highest among sub-groups) setting lacked optimal confidence in educating patients about treatment limitations or encouraging early treatment adherence. Respondents in France and Germany's community settings reported suboptimal confidence (27% in both countries) for encouraging treatment adherence beyond 12 months. Sub-optimal confidence explaining to patients how new evidence may change treatment options was reported by 27-29% of respondents (except for Germany academic, 14%; Table 2)Conclusions:
These data suggest the need to further the understanding of ONC/HEM of the importance of considering patients' preferences in treatment decisions in relapsed/refractory CLL/MCL. Qualitative research could help understand why ONC/HEMs may not systematically consider patient preferences in treatment decisions and educating for treatment adherence. This study also suggests building providers' confidence to foster treatment adherence among patients. Future initiatives could include continuing education activities and practical tools to incorporate patients' considerations in treatment choices and discussion, and encouraging providers to promote treatment adherence.
Disclosures
Shah:Lilly Oncology: Consultancy, Honoraria; Miltenyi Biotec: Consultancy, Research Funding; Incyte Corporation: Consultancy, Honoraria, Speakers Bureau; TG therapeutics: Consultancy; Bristol Myers Squibb: Consultancy; Kite Pharma: Consultancy; Epizyme: Consultancy; Novartis: Consultancy. Cymbalista:Roche, Janssen, Abbvie, AstraZeneca and Lilly: Consultancy. Dreyling:Amgen, Astra Zeneca, Gilead/Kite, Janssen, Lilly, Novartis, Roche: Honoraria; Astra Zeneca, Beigene, BMS/Celgene, Gilead/Kite, Janssen, Lilly/Loxo, Novartis, Roche: Consultancy; Abbvie, Bayer, BMS/Celgene, Gilead/Kite, Janssen, Roche: Research Funding. Fiacco:Eli Lilly Italia: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal